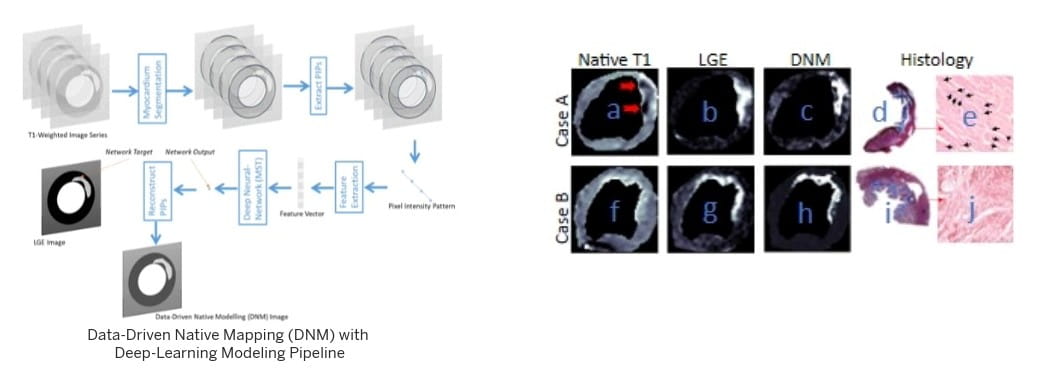
CIRC investigators study various forms of diseases that affect the heart muscle, with a major focus on ischemic heart disease (IHD), a class of heart disease that stems from reduced oxygen supply to the heart muscle. IHD is the most common cause of death in western countries.
Our scientists aspire to better understand heart disease from the standpoint of structural, compositional and functional changes to the heart in the early, progressive and terminal stages of the disease using an integrated multidisciplinary approach.
We conduct these studies using sophisticated imaging technologies, particularly MRI and PET, biomedical engineering methods, molecular immunopathological analyses, multi-omics and informatics.
Identifying biomarkers to predict progression of heart disease
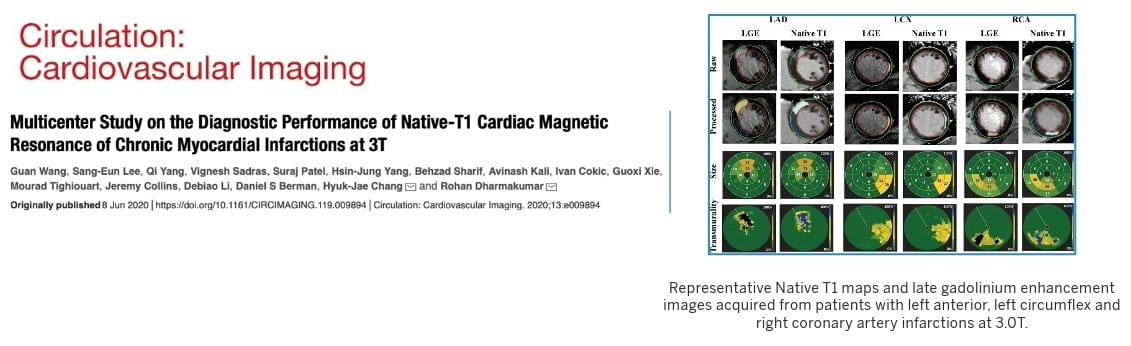
Funded by multiple agencies including the National Institutes of Health and the American Heart Association, CIRC scientists are investigating novel tools for early diagnosis of disease through the identification of biomarkers for non-invasive and reliable detection of myocardial injury in the progressive phase of disease advancement, that will aid in early diagnosis of disease timely intervention strategies.
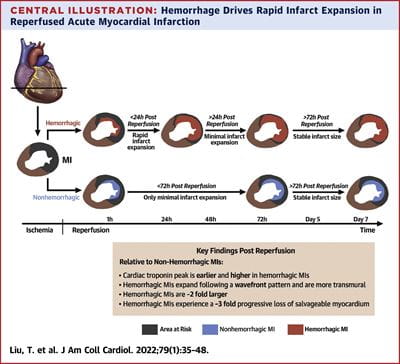
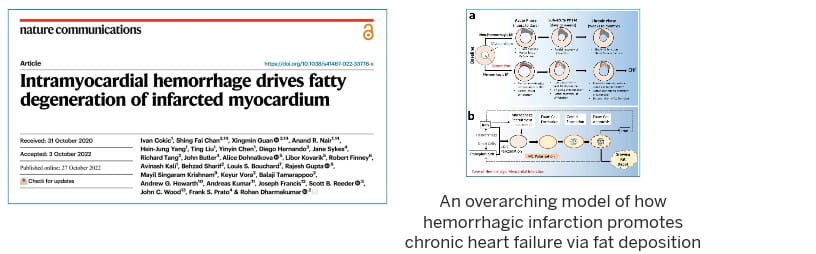
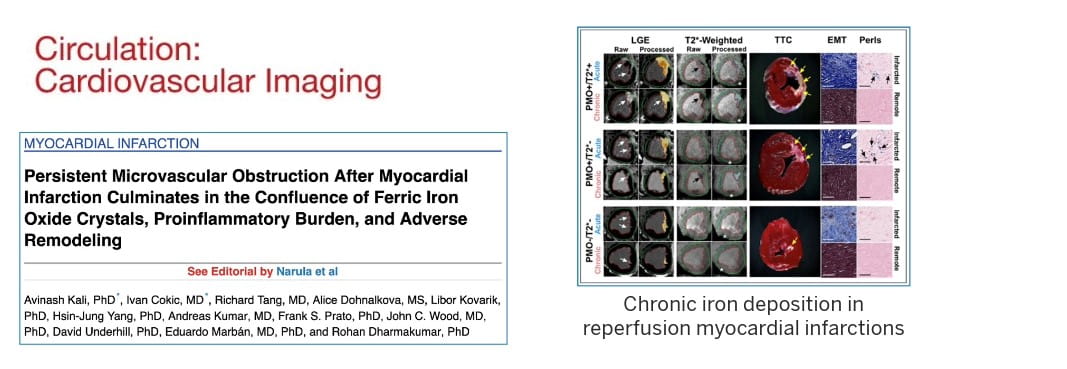
In November 2022, our landmark study published in Nature Communications showed that residual iron following heart attacks plays a causative role in chronic heart failure, through the formation of fatty tissues in the heart.
 That study formed a central basis in the development of the first ever clinical classification of heart attacks that can help inform clinicians on the best treatment options based on the stage of heart damage. This classification was officially adopted by the Canadian Cardiovascular Society in 2024 and has been well received by the international cardiovascular research community. It also led to the first-in-human FDA approved trial to treat hemorrhagic myocardial infarction.
That study formed a central basis in the development of the first ever clinical classification of heart attacks that can help inform clinicians on the best treatment options based on the stage of heart damage. This classification was officially adopted by the Canadian Cardiovascular Society in 2024 and has been well received by the international cardiovascular research community. It also led to the first-in-human FDA approved trial to treat hemorrhagic myocardial infarction.
In September 2025, another major milestone was achieved by our scientists enabling the detection of hemorrhagic myocardial infarction based on a blood test. This test is expected to open new opportunities for novel therapies for patients developing hemorrhagic myocardial infarction (the worst form of heart attacks) and to empower imaging approaches for deep tissue characterization.