The Cardiopulmonary Research Program (CRP) at the Krannert Cardiovascular Research Center studies the basic molecular underpinnings of heart and lung disorders using state-of-the-art precision medicine tools and techniques.
Led by Ankit A. Desai, MD, CRP is dedicated to understanding the mechanisms and genetics of pulmonary arterial hypertension (PAH), myocardial infarction(MI), lipid metabolism, and sickle cell cardiomyopathy (SCC), complemented by Dr. Desai’s active clinical practice serving patients with cardiopulmonary disorders for more than 19 years. The program employs genome-wide association study strategies to prioritize functional and preclinical studies of candidate genes that lead to the development of PAH, MI,and heart failure.
Pulmonary arterial hypertension is a type of high blood pressure that impacts the arteries in the lungs and the right side of the heart when blood vessels in the lungs become narrowed, blocked or damaged. This condition over time can lead to right heart failure.
Sickle cell disease is a genetic condition and type of anemia characterized by an abnormal shape of red blood cells that become blocked in blood vessels or break down, and cause harm to multiple organs including the heart.
CPR focuses on three major cardiovascular domains:
- Genetic mechanisms of pulmonary arterial hypertension (PAH) and right heart failure, which impacts the heart’s right ventricle, with a particular focus on endothelial cell signaling
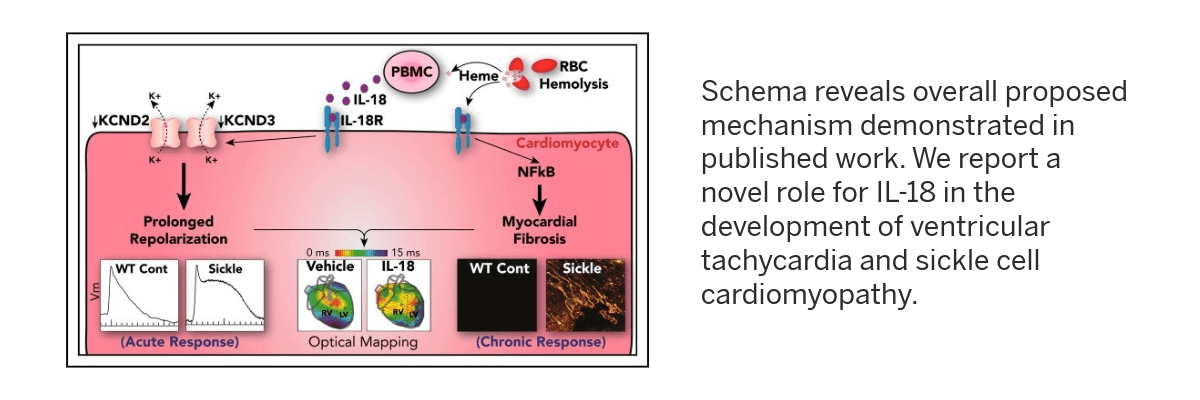
- Genetic mechanisms of inflammation-mediated ventricular tachycardia and cardiomyopathy in patients with sickle cell disease
- Novel molecular mechanisms of beta-blockers in myocardial infarction via beta-adrenergic independent signaling.
These areas reflect CPRs multi-disciplinary expertise across molecular biology, cellular signaling and animal physiology. Each domain is fundamentally grounded in omics-based platforms and studies of diverse populations and health disparities.