This is the final column in a four-part series from Taeho Jo, PhD, assistant professor of radiology and imaging sciences at the Indiana University School of Medicine, titled "AI in Medicine: From Nobel Discoveries to Clinical Frontiers."
Read Column 1: AI Innovation Through the Lens of the 2024 Nobel Prizes
Read Column 2: The Protein Folding Problem: The day AI unlocked a secret of life
Read Column 3: What is your 'Aha' moment?
Some memories remain vivid even after many years. The dementia patients I met 20 years ago are among them. During my master's studies in biomedical engineering, I had to install and test equipment that monitored patients at care facilities. It was frustrating that all our research team could do for these Alzheimer's patients was monitor them to prevent injuries and keep them from leaving restricted areas. Years later, around the time I finished my long PhD journey in computational biology, I suddenly realized that all those elderly patients I had met during my experiments had passed away. My heart ached knowing that while I earned my degree through them, I didn't know what they had gained from me. This experience of helplessness in the face of an incurable disease later had a profound influence on my decision to apply to Professor Andrew Saykin's group at Indiana University to research Alzheimer's, leaving behind my work in AI for protein structure prediction. This is the story behind the science. And this story is one of the three uniquely human qualities that AI can never possess.
Story: The human context behind the science
In my previous column, I explored three uniquely human abilities: story, seeking and sincerity. I believe the real warning about AI in our current era is not that AI will replace humans, but rather that we now face competition between those who use AI well and those who don't. The competitive advantage of those who excel at using AI depends on how appropriately and effectively they leverage these three distinctly human qualities that differentiate us from AI. Today, I want to demonstrate through Alzheimer's research how these three qualities can guide AI to help us overcome disease.
When I joined IU in 2018, Alzheimer's research hadn't progressed far beyond what had frustrated me at the medical school in Tokyo 20 years earlier. But 2023 and 2024 marked a turning point. The FDA approved Leqembi (lecanemab) and Donanemab, the first disease-modifying treatments offering real hope after decades of limited progress. But here's the challenge: these drugs work best when given early. They reduce amyloid plaques in the brain, but once neurons die, we can't bring them back. So we need to find people who have Alzheimer's pathology developing but don't have symptoms yet. This is difficult because early signs are subtle. When someone forgets a name or can't find the right word, most people think it's just normal aging. But Alzheimer's-related changes, especially amyloid accumulation, can occur in the brain for 15 years or more before serious memory problems appear. This long pre-symptomatic period is when early detection could make a difference, if we can identify who's at risk.
Seeking: Breaking boundaries with new questions
Given this critical need, we asked: "How can recent AI breakthroughs, like AlphaFold's revolutionary success, be used for early Alzheimer's risk assessment?"
AlphaFold showed us that AI could find patterns in biological data that traditional methods missed. We applied this lesson to Alzheimer's research. Inspired by this multi-modal strategy, we applied similar deep learning techniques across three biological data types: imaging, genomic, and metabolomic data to detect Alzheimer's pathology in its earliest stages.
First, we enhanced brain imaging analysis through pattern recognition. We trained our AI to learn tau accumulation patterns from people who eventually developed dementia, then used this knowledge to identify similar patterns in those still cognitively healthy. Using CNNs with explainable AI techniques, we could spot these high-risk patterns years before symptoms appear.
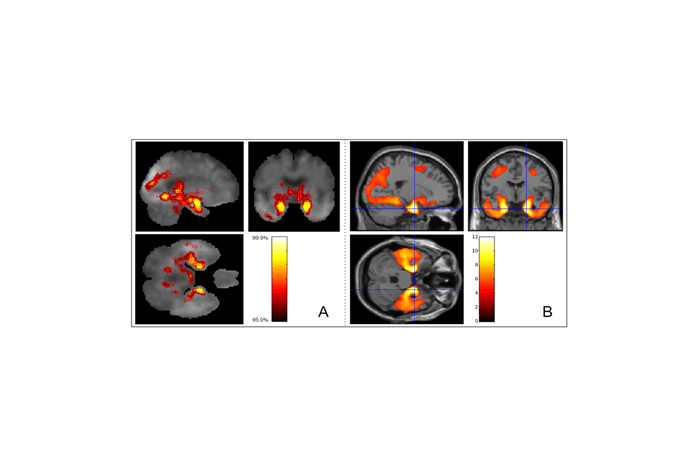
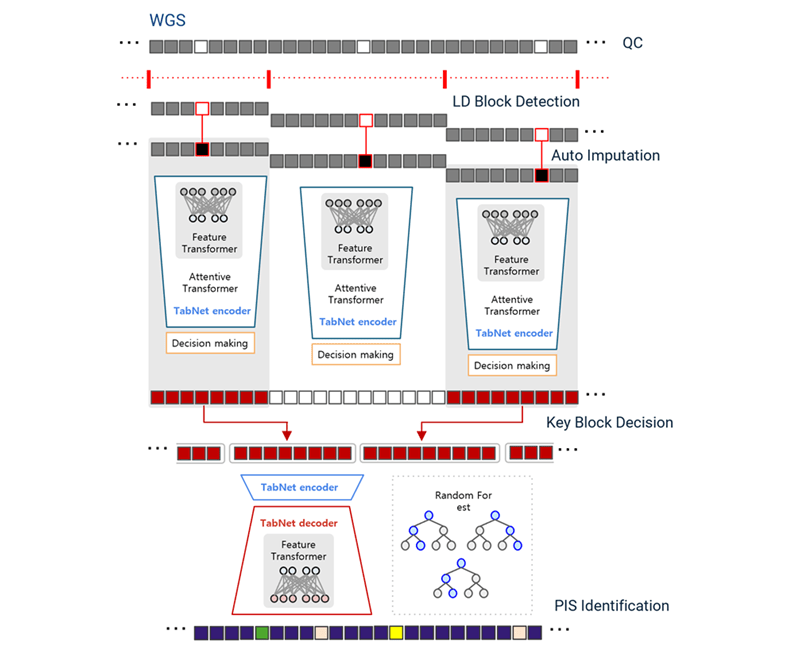
Second, we used genomics for earlier detection. Since genetic data is fixed at birth, it offers the earliest possible risk assessment. We developed DeepBlock, employing transformer-based architectures similar to those in modern language models, to decode AD-relevant patterns from whole genome sequences.
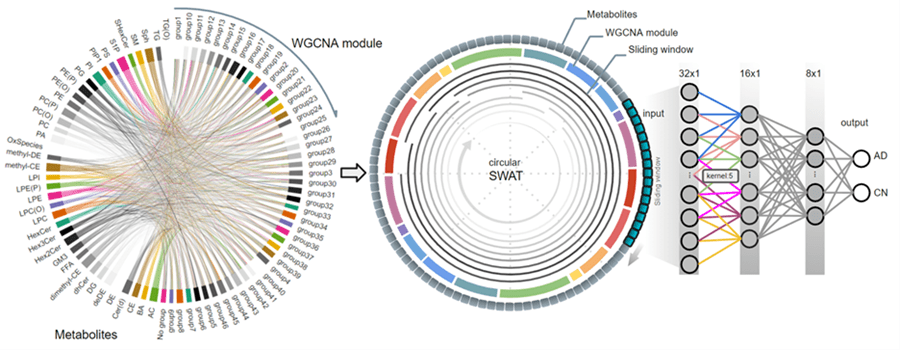
Third, we used metabolomics for its comprehensive biological information. Unlike static genetic data or expensive imaging, metabolomic profiles capture dynamic changes in brain metabolism as the disease progresses. Our c-SWAT analyzes these complex metabolic networks to detect early Alzheimer's signatures.
Sincerity: From lab to life through precision medicine
The ultimate goal of our AI research is personalized care for each individual facing Alzheimer's risk. Every person's journey with this disease is unique, shaped by their genetics, lifestyle and biology.
Our integrated approach creates a comprehensive picture for each patient. By combining tau patterns, genetic markers and metabolic signatures, we can understand where someone stands on the disease spectrum. This allows physicians to have informed conversations with patients about their specific risks and options. A 60-year-old with concerning metabolic changes might benefit from different interventions than someone with genetic risk but no current biological markers.
Making this work requires more than accurate predictions. The AI must translate complex data into insights that physicians can share with patients. Families need to understand what the results mean for their loved ones. This is the essence of sincerity in medical AI: ensuring our technology enhances the human connection between physicians and patients, providing them with the precise information needed for meaningful, personalized care decisions.
![[Figure] Multi-modal approach for early Alzheimer's disease detection through precision medicine](https://mc-34647c8d-0ad3-4e6c-832a-7092-cdn.azureedge.net/-/media/centers/alzheimers/edited-column-4-no-5.png?h=600&w=900&rev=5c5fd3127e104dafb8bc24206dc62916&hash=E1E6A3042EBDBE3E3A84818B19D6AB7A)
The path forward
