When sporadic tumor cells arise due to various genetic or epigenetic defects, the body’s immune surveillance system detects and destroys newly formed tumor cells, preventing them from growing and spreading. However, when immune surveillance is compromised, unchecked tumor cells or tumor stem cells proliferate, grow freely and spread to other organs. Investigating the molecular and cellular mechanisms involved in inflammatory signaling and immune regulation during tumorigenesis have been my primary research focus for the last twenty years. We have established multiple inflammation-induced spontaneous and tumor-bearing animal models that facilitate identification of immune molecules and myeloid-derived suppressive cells as targets and biomarkers for diagnosis, prognosis and immunotherapeutic treatment of various cancer forms, particularly non-small cell lung cancer (NSCLC). Several patents have been issued based on these significant findings.
Currently, we are focusing on two lines of research:
-
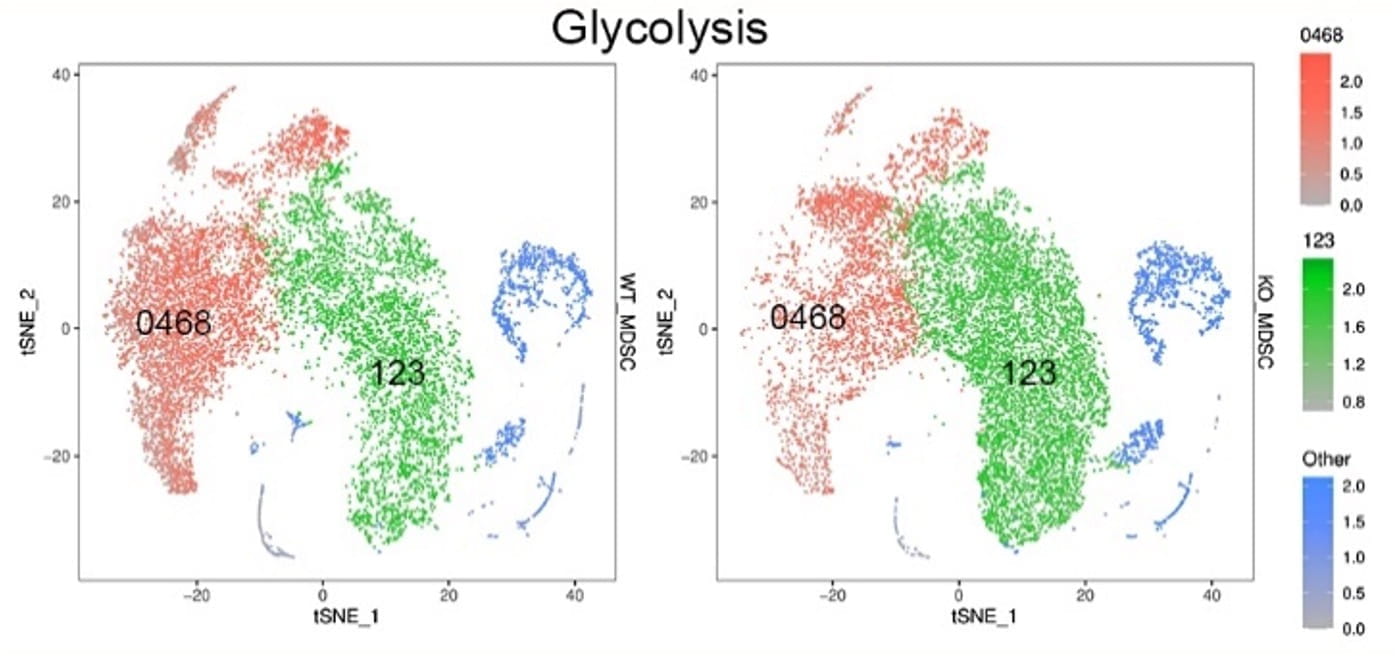
Anti-cancer immunity involves two basic steps: nonspecific innate immunity followed by antigen presenting cells (APCs) initiated-specific T cell adaptive immunity. CD11c+ dendritic cells (DCs) are specialized professional APCs capable of recognizing and presenting cancer-associated antigens to T cells through the major histocompatibility complex (MHC) molecules, leading to the activation of a specific immune response against cancer. However, the deficiency of lysosomal acid lipase (LAL), which hydrolyzes cholesteryl esters and triglycerides to generate free cholesterol and free fatty acids in the lysosomes, induces CD11c+ myeloid-derived suppressor cell (MDSC) expansion and promotes tumor growth and progression by creating an immunosuppressive environment that allows the cancer to evade immune surveillance. Therefore, CD11c+ cells possess dual functions depending on genetic defects and existing microenvironments: (a) antitumor APC/T cell stimulating function, and (b) protumor MDSCs /T cell suppressive function. We aim to identify novel molecules and pathways that control the transition between these two states of cancer immunity.
-
The lung serves as an interface for gas exchange between the air and blood, facilitating oxygen supply and carbon dioxide removal. However, imbalanced and excessive inflammation during respiratory cycles can lead to severe consequences, contributing to the pathogenesis of acute and chronic pulmonary remodeling and disorders such as emphysema/COPD and lung cancer. One commonly hyperactivated pro-inflammatory molecule during pulmonary inflammation is Signal Transducers and Activators of the Transcription 3 (Stat3). In our laboratories, we generated a doxycycline-controlled CCSP-rtTA/(tetO)7-Stat3C mouse model that overexpresses constitutively active form of Stat3C in alveolar type II (AT II) epithelial cells to assess the consequences of persistent Stat3 activation in the lung. In sequential steps, Stat3C overexpression in AT II epithelial cells leads to severe pulmonary inflammation, characterized by immune cell infiltration and upregulation of pro-inflammatory cytokines and chemokines. Consequently, spontaneous bronchoalveolar adenocarcinoma develops concurrently with emphysema, suggesting that AT II epithelial cells can serve as regional cancer stem cells of lung tumorigenesis. Transcriptome analysis of CCSP/Stat3C-overexpressing AT II epithelial cells identifies a panel of soluble secretory proteins encoded by the Stat3 downstream genes. Many of these secretory proteins are upregulated in human lung carcinomas and COPD with a history of smoking at both mRNA and protein levels, serving as authentic biomarkers for lung cancer diagnosis and prognosis. Our research aims to systematically characterize the functional roles of Stat3C-initiated immunosuppression in lung tumorigenesis.