The laboratory was the first to report that peripheral nerve injury results in the upregulation of both the chemokine, monocyte chemoattractant protein-1 (MCP1/CCL2) and its cognate receptor, CCR2. More importantly, that the use of a receptor antagonist, CCR2 RA [R], could elicit the reversal of neuropathic pain behavior. The White Lab also reported that other chemokine/receptor interactions could modulate chronic pain states in demyelinating peripheral neuropathy, painful neuropathy due to therapeutic drugs for HIV and bone cancer. More recent studies have examined the role that TRPV1, TRPA1, TRPC6, voltage-gated ion channels (NaV1.6/1.7, CaV2.2) toll-like receptor 4 (TLR4) and receptor of advanced glycation end-products (RAGE) play in the persistence of chronic pain states due to bone fracture, peripheral nerve injury, mild traumatic brain injury and opioid-induced hyperalgesia.
White Lab
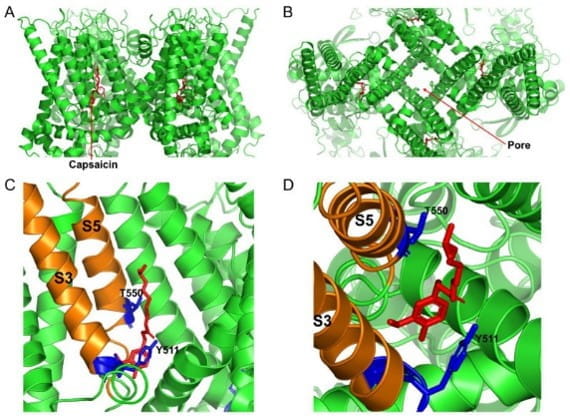
Cryo-EM structure of the open capsaicin-bound rat TRPV1 channel at 48 °C. (A) shows the side view of the TRPV1 channel, and (B) shows the bird’s eye view of the channel. (C,D) show the magnified views of the capsaicin binding site from the side and from the top, respectively. The key residues that are important for coordinating capsaicin are shown in blue. S3 and S5 transmembrane domains are colored in orange, and capsaicin molecules are shown as red sticks. (Munjuluri et al., Cells 2021 Dec 22;11(1):18 PMID: 35011580)
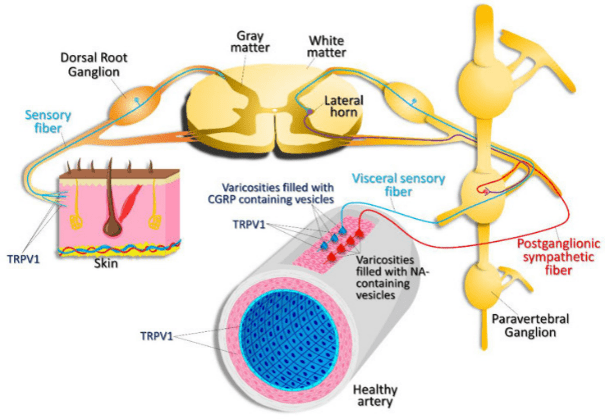
Expression pattern of TRPV1. TRPV1 channels are expressed in nociceptive, sensory nerve fibers innervating the skin and visceral sensory nerve fiber endings, smooth muscle, and endothelial cells within the vascular wall. TRPV1 activation in visceral sensory nerve endings leads to calcitonin gene related peptide (CGRP) release. CGRP then acts on vascular smooth muscle cells, causing vasodilation. Activation of postganglionic sympathetic nerve fibers results in noradrenaline (NA) release, which results in vasoconstriction. (Munjuluri et al., Cells 2021 Dec 22;11(1):18 PMID: 35011580)
Several of our pre-clinical studies have also translated into clinical observational and interventional studies. These projects include biomarkers of (i) subjects with mild traumatic brain injury who develop chronic headache; (ii) subjects with polytrauma alone or in combination with mild to moderate traumatic brain injury and (iii) subjects with spinal cord injury suffering with neuropathic pain. Additionally, an FDA-approved drug identified from one of the preclinical studies is now in a NIH RO1-sponsored phase I clinical trial for a novel use in patients suffering from pain due to chronic pancreatitis. These outcomes, achieved through extensive collaboration with clinical and basic scientists, demonstrate the translational nature of our research.
Current Research Funding
NIH/NIDDK R01DK132709 (White, MPI)
Safety, tolerability, and dose limiting toxicity of lacosamide in patients with painful chronic pancreatitis
2022-2026
NIH NIAMS R01AR083130 (White, Contact MPI)
Novel treatments of fracture repair and bone pain
2024-2029
US Department of Army, Chronic Pain Management Research Program, CP220068 (White, PI)
The Prevalence of Neuropathic Pain Pathophysiology Associated with Ankle Fracture
2023-2027
US Department of Veterans Affairs, RR&D MERIT, I01RX004297 (White, PI)
Novel treatments of chronic pain due to repetitive mild traumatic brain injury
2023-2027
Indiana Spinal Cord & Brain Injury Research Grant, (White, co-I)
TAK242 for the prevention of posttraumatic epilepsy and cognitive decline in a novel mouse model of repeated mild traumatic brain injury
2025-2027
Department of Veterans Affairs, RR&D MERIT (White, co-I)
Treatments for Alleviating Repetitive Traumatic Brain Injury-Associated Acceleration of Atherosclerosis
2026-2030
Recent Publications
Naugle KM, Nguyen T, Smith JA, Saxe J, White FA. Racial differences in pain-related outcomes following mild traumatic brain injury. Journal of Neurotrauma, 2023 Aug;40(15-16):1671-1683.
PMID: 36565020
Smith JA, Nguyen T, Karnik S, Davis BC, Al-Juboori MH, Kacena MA, Obukhov AG, White FA. Repeated Mild Traumatic Brain Injury in Mice Elicits Long Term Innate Immune Cell Alterations in Blood, Spleen, and Brain. Journal of Neuroimmunology, 2023 May 16;380:578106.
PMID: 37245410
Smith JA, Nguyen T, Davis BC, Lahiri D, Hato T, Obukhov AG, White FA. Propranolol Treatment During Repetitive Mild Traumatic Brain Injuries Induces Pronounced Transcriptomic Changes in the Bone Marrow of Mice. Frontiers of Neuroscience, 2023 Sep 12;17:1219941.
PMID: 37817806
Nguyen T, Nguyen NN, Cochran AG, Smith JA, Al-Juboori M, Brumett AM, Saxena S, Talley S, Campbell EM, Obukhov AG, White FA. Repeated Closed-Head Mild Traumatic Brain Injury Induced Inflammation is Associated with Nociceptive Sensitization. Journal of Neuroinflammation 2023 Aug 27;20(1):196
PMID: 37635235
Talley S, Nguyen T, Ye LV, Valiauga R, DeCarlo J, Mustafa J, Cook B, White FA, Campbell EM. Characterization of age associated inflammasome activation reveals tissue specific differences in transcriptional and post-translational inflammatory responses. Immunity and Ageing 2024 Sep 10;21(1):60. doi: 10.1186/s12979-024-00462-z.
PMID: 39256821
Bhagar R, Le-Niculescu H, Corey SC, Gettelfinger AS, Woods C, Schmitz M, Ebushi A, Matei E, Mullen J, Kurian SM, Shekhar A, White FA, and Niculescu AB Next-Generation Precision Medicine for Pain. Resubmitted to Molecular Psychiatry, Accepted, July 14, 2025.
Nguyen T, Naugle KM, Leslie K, Fletcher M, Arellano HD, Bahdanovich A, Garriga A, Hill LC, Natoli RM, White FA. The Prevalence of Neuropathic Pain Pathophysiology Associated with Ankle Fracture. PLoS 1; Accepted, July 11, 2025.
Research Team

Jared A. Smith
MD/PhD Graduate Student

Tyler Nguyen, PhD
Postdoctoral Fellow

Geunjoo Choi, MD, PhD
Visiting Associate Professor in Anesthesia

Mohammed Juboori, MS
Research Assistant

Natalie Taylor, MS
Research Assistant
