Research Projects
Targeting Sleep and Anxious–Depressive Symptoms as Modifiable Risk Factors in Alzheimer’s Disease
This research addresses a critical gap in Alzheimer’s disease (AD) science by reframing anxious–depressive symptoms (ADS) and sleep disturbances (SD) not merely as clinical outcomes but also potential modifiable risk factors contributing to AD pathogenesis.
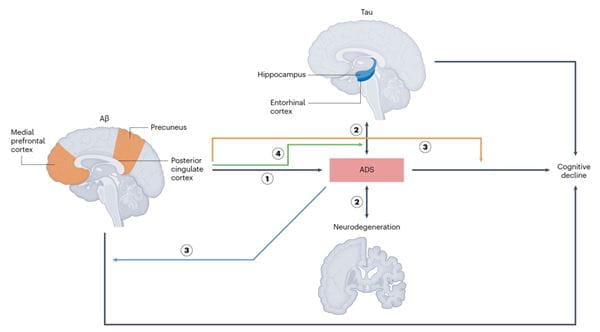
ADS-facilitated AD progression model
In an invited review in Nature Mental Health, we systematically examined how ADS and SD interact with core AD biomarkers — amyloid-β, tau and neurodegeneration — within the AT(N) research framework. We proposed integrative models illustrating how these symptoms may both reflect and drive neuropathological changes, influencing cognitive trajectories even in the preclinical and prodromal stages of AD.
This work provides a biologically informed rationale for targeting ADS and SD in AD prevention and intervention strategies and emphasizes the need for precsion medicine approaches that align symptom profiles with biomarker-defined disease states. We advocate for a paradigm shift that integrates neuropsychiatric phenotyping into early detection pipelines to improve patient outcomes and reduce disease burden through timely, targeted interventions.
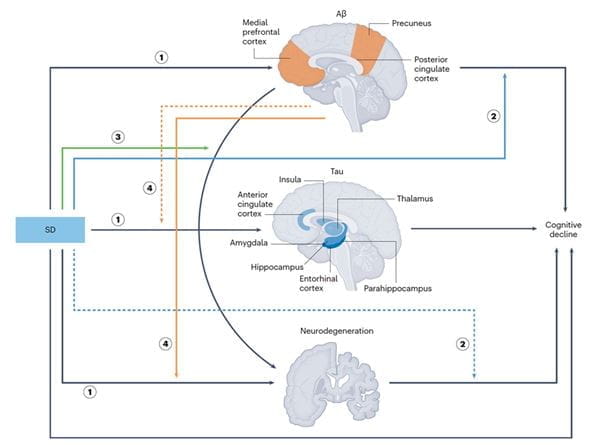
SD-facilitated AD progression model